Diabetes affects over 500 million people worldwide, and insulin is life-saving for millions. But the cost? It’s crushing. A vial of branded insulin can run $250 to $450 in the U.S. - and many patients skip doses because they can’t afford it. Enter insulin biosimilars: nearly identical versions of brand-name insulins, priced 15% to 30% lower, with the same safety and effectiveness. They’re not generics. They’re not copies. They’re complex biological products made in living cells, and getting them right takes years of science. Yet despite their promise, adoption is slow - especially in the U.S. Why? And which ones are actually out there?
What Makes Insulin Biosimilars Different From Generics
Generic drugs are simple. They’re exact chemical copies of brand-name pills. Take metformin - whether it’s branded or generic, the molecule is identical. Insulin? Not even close. Insulin is a protein made by living cells - yeast or bacteria - not synthesized in a lab. That means even tiny changes in the manufacturing process can affect how it behaves in your body. That’s why insulin biosimilars aren’t called generics. They’re called biosimilars.To get approved, a biosimilar must show it’s highly similar to the original insulin - no clinically meaningful differences in safety, purity, or potency. That means running dozens of lab tests, animal studies, and human trials. The FDA and EMA don’t just look at the final product. They check how it’s made, how it’s stored, how it behaves in the bloodstream. A generic drug might need one small study. An insulin biosimilar needs three or four clinical trials.
And here’s the kicker: even after approval, some doctors and patients still worry. They think, “If it’s not the same, what if it doesn’t work?” But the data says otherwise. Multiple studies - including those published in Diabetes, Obesity and Metabolism - show that biosimilar insulins like Basaglar and Semglee perform just like Lantus in controlling blood sugar. A 2025 survey found 68% of patients switching to biosimilars saw no change in their A1C or side effects. For many, it’s the first time they can afford insulin without rationing.
Market Examples: Which Biosimilar Insulins Are Available?
As of early 2026, six insulin biosimilars are approved in the European Union. In the U.S., the list is smaller but growing fast. Here are the key players:
- Basaglar - Biosimilar to Lantus (insulin glargine). Approved in 2016. Made by Eli Lilly and Boehringer Ingelheim. One of the most widely used.
- Semglee - Also a biosimilar to Lantus. Approved in 2021. Developed by Biocon and marketed by Viatris. Noted for its lower price point.
- Admelog - Biosimilar to Humalog (insulin lispro). Approved in 2019. Offers rapid-acting control.
- Lusduna - Another Lantus alternative. Approved in 2022. Used in Europe and parts of Asia.
- Rezvoglar - Approved in 2023. Also targets insulin glargine. Marketed by Eli Lilly.
- Fiasp biosimilar - Expected in 2026. Will compete with Novo Nordisk’s fast-acting insulin.
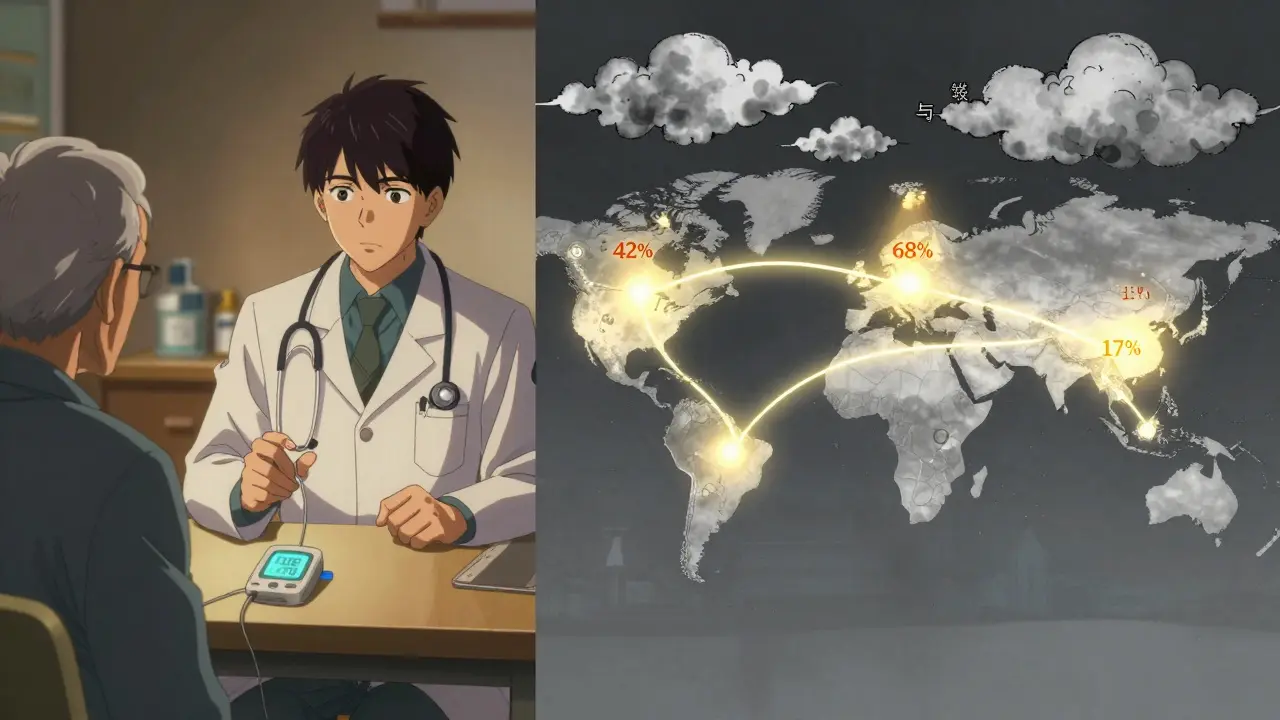
These aren’t just lab curiosities. In India, where 77 million people have diabetes, biosimilars make up nearly half of insulin prescriptions. Dr. Arjun Patel, an endocrinologist in Mumbai, says his patients’ out-of-pocket costs dropped from $120/month to $35/month after switching. In Germany, biosimilar insulin use jumped from 10% to 42% in five years thanks to government incentives.
But in the U.S., adoption is stuck. Why? One reason: Sanofi still sells Lantus under two names - the branded version and a cheaper, unbranded version. That keeps prices high and confuses payers. Another reason: many insurers still require prior authorization for biosimilars, even though they’re proven safe.
Why Adoption Is Slower Than in Other Therapies
Think about biosimilars in oncology. Drugs like bevacizumab biosimilars now make up over 80% of the market in the U.S. Why? Because cancer patients and doctors don’t have time for hesitation. They need treatment now. Insulin? It’s different.
First, patients are used to their brand. If you’ve been on Lantus for 10 years and your blood sugar is stable, why switch? Even if the science says it’s safe, the fear of change is real. Second, automatic substitution is rare. In the U.S., only 17 states let pharmacists swap a branded insulin for a biosimilar without the doctor’s approval. In the EU, it’s automatic. In Australia, it’s allowed with documentation. In the U.S., it’s a patchwork.
Third, manufacturers of original insulins use clever tactics. Sanofi’s dual pricing strategy - selling Lantus under two names - makes it hard for payers to compare true costs. Eli Lilly and Novo Nordisk have extended patents on delivery pens and formulations to delay biosimilar entry. And while the FDA requires biosimilars to be proven safe, it doesn’t automatically label them as “interchangeable.” Only one insulin biosimilar - Semglee - has that designation in the U.S. That means a pharmacist can’t swap it without a doctor’s okay.
Meanwhile, doctors get conflicting messages. Some clinics push biosimilars hard. Others avoid them entirely. A 2025 survey of U.S. endocrinologists found that 43% had never prescribed a biosimilar insulin. The main reason? “I don’t feel confident about switching patients without more data.” But the data is there. The hesitation is psychological, not scientific.
Real Patient Experiences: Successes and Setbacks
Stories from patients tell the real story.
On the American Diabetes Association forum, a user named DiabetesWarrior87 wrote: “Switched to Basaglar. My A1C dropped from 7.8 to 7.2. My monthly cost went from $450 to $90. I can finally sleep at night.”
But another user on Reddit shared: “My doctor switched me to a biosimilar without telling me. Two weeks later, I was having lows at 3 a.m. I had to switch back.”
That second story isn’t rare. About 22% of patients report needing a small dose adjustment after switching. That’s not because the biosimilar is unsafe - it’s because insulin sensitivity can vary slightly between products. A patient who’s been on Lantus for years might need 0.5 units less of Basaglar to get the same effect. That’s why the American Association of Clinical Endocrinologists recommends a 3- to 6-month transition period with close glucose monitoring.
Most patients don’t need to change their routine. But they do need to be warned. Too often, switching happens quietly - at the pharmacy, without a conversation. That’s where trust breaks down.
Regulatory Differences: U.S. vs. Europe vs. Australia
The rules vary wildly.
In the European Union, the EMA approves biosimilars and considers them interchangeable by default. No extra studies needed. Pharmacies can substitute freely. Germany, France, and Spain have aggressive policies that push biosimilars to 60%+ market share.
In the United States, the FDA requires a separate “interchangeable” designation. Only Semglee has it so far. That means even if a biosimilar is approved, a pharmacist can’t swap it unless the doctor says so - and only in states that allow it. CMS reimburses biosimilars at ASP + 8%, which helps providers stay profitable. But insurance formularies still favor brand names.
In Australia, the Therapeutic Goods Administration (TGA) takes a middle path. Biosimilars are approved after strict testing, but substitution is allowed only with patient consent and documentation. The government subsidizes them under the PBS (Pharmaceutical Benefits Scheme), making them affordable - often under $30 per vial. That’s why uptake is rising fast.
That’s the problem: there’s no global standard. A biosimilar approved in the EU might not even be reviewed in the U.S. or Australia. Manufacturers have to run separate trials. That slows everything down.
What’s Coming Next: New Products and Delivery Systems
The next wave of insulin biosimilars is already in the pipeline. By late 2026, biosimilars for Toujeo (ultra-long-acting insulin) and Tresiba (another long-acting insulin) are expected to launch. These will compete with Novo Nordisk’s high-priced, long-lasting insulins - products that currently have no competition.
And it’s not just about the insulin. Manufacturers are bundling biosimilars with smarter delivery systems. 78% of companies are now investing in connected pens, apps that track doses, and refill reminders. One company in China is testing a biosimilar insulin that comes in a smart patch - no injections needed. That could change everything for kids and needle-phobic adults.
The market is projected to grow from $3.2 billion in 2025 to $6.7 billion by 2032. That’s an 18% annual growth rate - nearly triple the pace of the overall biosimilars market. Why? Because the need is urgent. In China, 141 million people have diabetes. In India, 77 million. In the U.S., 38 million. And insulin prices haven’t dropped - until now.
What Patients and Providers Should Do Now
If you’re on insulin and paying more than $100 a month:
- Ask your doctor: “Is there a biosimilar version of my insulin?”
- Check your insurance formulary. Many plans now list biosimilars as preferred options.
- Don’t let your pharmacy switch you without telling you. Know what you’re getting.
- Monitor your blood sugar closely for the first 3 months after switching.
- Report any changes - even small ones - to your provider.
If you’re a provider:
- Use the Biologics Prescribers Collaborative’s 2025 Clinical Guide. It details switching protocols for 12 biosimilars.
- Don’t assume patients know the difference between biosimilar and generic. Explain it simply.
- Start with new patients. It’s easier to begin with them than to switch someone stable.
- Advocate for state-level policies that allow pharmacist substitution.
Insulin biosimilars aren’t perfect. But they’re the best shot we’ve had in decades to make insulin affordable without sacrificing safety. The science is solid. The cost savings are real. The only thing holding us back is fear - and bureaucracy.
Are insulin biosimilars safe?
Yes. Every insulin biosimilar approved by the FDA or EMA has passed rigorous testing showing no clinically meaningful differences in safety or effectiveness compared to the original product. Studies involving over 15,000 patients confirm they work just as well for controlling blood sugar and have similar rates of side effects like hypoglycemia.
How much cheaper are insulin biosimilars?
They typically cost 15% to 30% less than the brand-name version. In the U.S., a vial of Lantus might cost $450, while Basaglar or Semglee can be as low as $90 to $120 with insurance. In countries like India and Australia, prices can be 60% to 70% lower.
Can I switch from my current insulin to a biosimilar?
Yes, but it should be done under medical supervision. Most patients can switch safely, but a small percentage (about 22%) may need a slight dose adjustment during the first few weeks. A 3- to 6-month monitoring period is recommended to ensure stable blood sugar levels.
Why aren’t biosimilars used more in the U.S.?
Several reasons: lack of automatic substitution (only 17 states allow it), insurance formularies favoring brand names, physician hesitation due to unfamiliarity, and pricing strategies by original manufacturers that keep branded insulin competitive. Also, only one insulin biosimilar (Semglee) has the FDA’s “interchangeable” designation, limiting pharmacy substitution.
Do biosimilars work the same for type 1 and type 2 diabetes?
Yes. Clinical trials for insulin biosimilars include both type 1 and type 2 diabetes patients. The insulin molecule works the same way regardless of diabetes type - it replaces what the body can’t make or use properly. Safety and effectiveness are proven across both populations.








Chuck Dickson January 18, 2026
Just switched my mom to Semglee last month-her A1C stayed the same, and her copay dropped from $380 to $85. No more choosing between insulin and groceries. If you’re on brand-name insulin and paying over $100 a vial, you’re literally being scammed. Biosimilars aren’t ‘cheap’-they’re just not overpriced anymore.
Naomi Keyes January 19, 2026
Wait-so you’re saying that the FDA approved these… but only ONE is ‘interchangeable’? That’s not science-that’s corporate lobbying disguised as regulation. And don’t get me started on how pharmacies are allowed to swap without consent-this is medical autonomy being stripped away under the guise of ‘cost-saving’!
Andrew Qu January 20, 2026
Naomi-your concern about autonomy is valid, but the data shows no safety risk. The real issue is communication. If your pharmacist swaps your insulin without telling you, that’s a system failure-not a biosimilar failure. Doctors and pharmacists need to talk to patients. Not hide behind forms.
Dayanara Villafuerte January 21, 2026
Y’all are overcomplicating this 😅. Biosimilars = same medicine, 1/4 the price. If your doctor’s scared, ask them to read the JAMA study from last year. Or better yet-ask them why they still prescribe Lantus when Basaglar costs less than a Starbucks latte. 💸
Zoe Brooks January 22, 2026
I used to think biosimilars were ‘second-rate’-until I saw my brother’s insulin bill drop from $500 to $110. Now I’m the one pushing my whole family to switch. It’s not magic. It’s just fairness. Why should life-saving medicine be a luxury? 🤷♀️
Andrew Short January 23, 2026
Let’s be real-this whole ‘biosimilar’ thing is a pharma scam to kill American innovation. The FDA approves these because they’re bribed by foreign manufacturers. Lantus was developed by German scientists with decades of R&D. Now some Indian lab copies it, sells it for $90, and we’re supposed to cheer? Pathetic.
Danny Gray January 23, 2026
So you’re saying the real enemy isn’t insulin cost-it’s the fear of change? That’s poetic. But also… lazy. If we’re so scared of switching insulins, why don’t we just make insulin free? Why does a molecule have to be patented like a smartphone? The system isn’t broken-it’s designed this way.
Andrew McLarren January 25, 2026
While the economic argument for biosimilars is compelling, one must not overlook the rigorous regulatory framework underpinning their approval. The FDA’s requirement for analytical, nonclinical, and clinical comparability is not a mere formality-it is a safeguard against biological variability that could, in rare cases, manifest as altered pharmacokinetics. The data, while overwhelmingly favorable, must be interpreted with scientific rigor, not emotional appeal.
kenneth pillet January 26, 2026
my doc switched me to basaglar last year. no issues. my sugar’s better. i pay less. why is this even a debate?
Kristin Dailey January 26, 2026
Stop giving in to foreign medicine. Buy American insulin. Support U.S. jobs. This isn’t about cost-it’s about sovereignty.
christian Espinola January 28, 2026
Wait. You said ‘Semglee is the only interchangeable one.’ But didn’t the EMA approve all biosimilars as interchangeable? So why does the FDA lag? Because they’re under the thumb of Big Pharma. And guess who owns the FDA advisory board? Hint: It’s not the patients. It’s the same CEOs who raised insulin prices 1000% since 2001.
Robert Cassidy January 30, 2026
They want you to think this is about money. It’s not. It’s about control. Who owns your body? The FDA? The pharmacy? The multinational conglomerate that owns the patent? Biosimilars are the Trojan horse. Once you accept ‘close enough,’ what’s next? ‘Close enough’ vaccines? ‘Close enough’ chemo? They’re normalizing compromise. And you’re cheering.
Tyler Myers January 31, 2026
Y’all are forgetting the REAL issue: insulin was never supposed to cost this much. It was invented in 1921. The patent was sold for $1. Now it’s a $450 monopoly. Biosimilars? Just a band-aid. We need price caps. We need public manufacturing. We need to treat medicine like a human right-not a stock ticker.
Jodi Harding February 1, 2026
They spent 20 years fighting this. Now they’re calling it ‘safe.’ Funny how ‘safe’ only matters after the profit’s been extracted.