
Search
Similar Posts
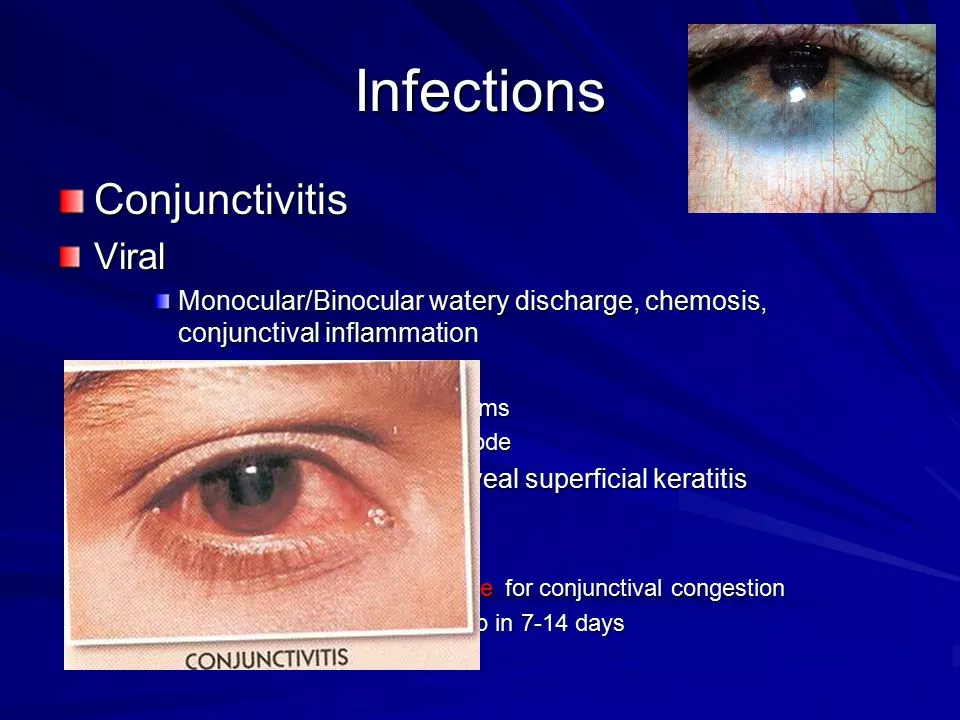
The Importance of Loteprednol in Ocular Inflammation Treatment
In my latest blog post, I discussed the significance of Loteprednol in treating ocular inflammation. Loteprednol is a corticosteroid that plays a crucial role in managing various eye conditions, such as allergic conjunctivitis, uveitis, and post-operative inflammation. I was amazed to learn how this medication effectively reduces inflammation and alleviates symptoms, providing relief to countless patients. Furthermore, Loteprednol is known for its reduced risk of side effects compared to other steroids, making it a safer choice in many cases. Overall, I truly believe that Loteprednol is a game-changer in the realm of ocular inflammation treatment.
Prochlorperazine for Migraine Relief: What You Need to Know
As someone who suffers from migraines, I recently discovered Prochlorperazine as a potential treatment option. This medication, commonly used to treat nausea and vomiting, has been found to help with migraine relief as well. If you're considering Prochlorperazine for your migraines, it's important to consult with your doctor to ensure it's the right choice for you. Side effects may occur, so it's crucial to be well-informed before starting any new medication. Overall, Prochlorperazine could be a game-changer for those of us constantly battling migraines.
The Effect of Telmisartan on Drug-Induced Hypertension
In my latest blog post, I've explored the effects of Telmisartan on drug-induced hypertension. Telmisartan, an angiotensin receptor blocker (ARB), has been shown to effectively lower blood pressure in patients suffering from this condition. Through my research, I've found that Telmisartan not only helps control hypertension, but also offers additional benefits such as reducing inflammation and oxidative stress. Furthermore, this medication has a lower risk of side effects compared to other ARBs. I highly recommend giving it a read to learn more about how Telmisartan can be a game-changer for those dealing with drug-induced hypertension.
How Breastfeeding Prevents and Treats Diaper Rash
Explore how breastfeeding protects against diaper rash, the science behind it, practical prevention steps, and treatment tips for irritated baby skin.
Ledipasvir: Tackling the Stigmas of Hepatitis C Treatment
Hepatitis C often carries stigma, adding a layer of stress for those seeking treatment. Ledipasvir shines a light on a more positive path with its effectiveness and more manageable regimen. This article explores how Ledipasvir has revolutionized treatment options, addressing common misconceptions and reassuring patients with practical advice. Learn how this approach is changing lives, making Hepatitis C less daunting.
Random Posts
Prochlorperazine: A Comprehensive Guide for Patients and Caregivers
Well, folks, let's dive headfirst into the world of Prochlorperazine, a superstar medication that aids in calming the stormy seas of nausea and vomiting. Now, if you or a loved one has ever felt like a seasick sailor on dry land, this guide is going to be your treasure map. It's a one stop shop for everything you need to know about this medicine, from what it does in the body, to the potential side effects, and even how to use it safely. By the end of our little journey, you'll be a Prochlorperazine pro! So, buckle up and get ready for a wild ride through the fascinating world of pharmaceuticals.
Antihistamines and Glaucoma: What You Need to Know Before Taking Allergy Medication
Certain allergy medications can trigger sudden, sight-threatening eye pressure spikes in people with narrow-angle glaucoma. Learn which antihistamines are dangerous, what’s safe to take, and how to protect your vision.
How to Safely Buy Viagra Online: Your 2025 Guide to Getting Prescription ED Medication
Discover how and where to buy Viagra online safely in 2025. Get practical steps, spot legit pharmacies, and avoid risky sites. Read this essential guide before you shop.
Risperdal vs Other Antipsychotics: How They Stack Up
A clear, side‑by‑side look at Risperdal versus other antipsychotics, covering how it works, key alternatives, costs, side effects, and tips for choosing the right medication.
Asthma and COPD Medications: Key Interactions and Safety Risks You Need to Know
Asthma and COPD medications can interact dangerously with common drugs like opioids, NSAIDs, and anticholinergics. Learn which combinations to avoid and how to protect your breathing.







Matt Cress May 14, 2023
Loteprednol? Oh great, another eye drop to add to my collecion.
Andy Williams May 20, 2023
Loteprednol possesses a unique corticosteroid profile, offering anti‑inflammatory efficacy while minimizing intraocular pressure elevation. Its molecular structure reduces systemic absorption compared with traditional steroids.
Paige Crippen May 25, 2023
One has to wonder why big pharma pushes Loteprednol so aggressively; perhaps it’s a stepping stone to more invasive ocular therapies concealed from the public.
sweta siddu May 31, 2023
Hey folks! 😊 Loteprednol is actually pretty cool for treating eye inflammation because it works fast and usually has fewer side effects. It’s a great option when you need quick relief! 🌟
Ted Mann June 6, 2023
While the clinical data on Loteprednol is compelling, it also invites deeper reflection on how we, as a society, tend to medicate discomfort rather than address underlying ocular health determinants. The drug’s targeted action exemplifies our preference for precision over holistic care.
Brennan Loveless June 11, 2023
Loteprednol may be marketed as “safer,” but let’s be real – our own medical research could produce comparable treatments without relying on foreign pharmaceutical giants.
Vani Prasanth June 17, 2023
Interesting point! While domestic research is valuable, collaborating internationally on drugs like Loteprednol can accelerate innovation and ensure patients get the best care possible.
Maggie Hewitt June 23, 2023
Loteprednol-because apparently we need a new name for “eye steroid” to make it sound fancy.
Mike Brindisi June 28, 2023
Loteprednol works fast it’s cheap and it saves you trips to the doctor
Steven Waller July 4, 2023
For anyone new to ocular pharmacology, Loteprednol is a soft steroid that offers anti‑inflammatory benefits with a lower risk of raising intraocular pressure, making it a suitable first‑line option.
Puspendra Dubey July 9, 2023
Yo! Loteprednol is like the superhero of eye drops 🦸♂️ – swoops in, calms the inflammation, and barely leaves a scar. #EyeCare
Shaquel Jackson July 15, 2023
Loteprednol? Sure, if you’re into paying extra for brand names. 🙂
Tom Bon July 21, 2023
In reviewing the pharmacodynamics of Loteprednol, one notes its reduced glucocorticoid receptor affinity, which correlates with a decreased incidence of steroid‑induced ocular hypertension.
Clara Walker July 26, 2023
Loteprednol’s approval timeline raises questions about regulatory capture; however, the data does support its efficacy, so dismissing it outright may be premature.
Jana Winter August 1, 2023
It is imperative to note that many lay discussions about Loteprednol suffer from grammatical inaccuracies and a lack of precise terminology; proper discourse demands exact language.
Linda Lavender August 7, 2023
Loteprednol, a relatively recent addition to the ophthalmic pharmacopeia, embodies a nuanced evolution in the therapeutic management of ocular inflammation, a condition that, despite its superficial appearance, can precipitate profound visual impairment if left inadequately addressed. Its molecular architecture, characterized by a unique esterified side chain, confers a selective affinity for glucocorticoid receptors, thereby attenuating the cascade of pro‑inflammatory cytokines while sparing the delicate trabecular meshwork from excessive pressure‑inducing effects. The clinical trials that underpinned its FDA approval were meticulously designed, encompassing a diverse patient cohort that ranged from postoperative cataract extraction cases to chronic uveitic presentations, thereby ensuring external validity across a spectrum of inflammatory etiologies. Moreover, the pharmacokinetic profile of Loteprednol reveals rapid ocular surface penetration followed by swift systemic clearance, a feature that mitigates the systemic adverse events commonly associated with more potent corticosteroids. In comparative studies, the incidence of intraocular pressure spikes was demonstrably lower than that observed with prednisolone acetate, a benchmark drug that has hitherto dominated the therapeutic algorithm. This safety margin has engendered a paradigm shift wherein clinicians feel empowered to prescribe Loteprednol with greater confidence, even in patients with pre‑existing glaucoma risk factors. Nevertheless, it would be remiss to overlook the economic considerations, as the branded formulations command a premium price point that may pose accessibility challenges for underinsured populations. The advent of compounding pharmacies offering generic equivalents has partially alleviated this burden, yet regulatory oversight of such preparations varies considerably across jurisdictions. From a mechanistic standpoint, the drug’s anti‑angiogenic properties have sparked ancillary research into its potential utility beyond conventional inflammation, including experimental applications in corneal neovascularization. Such investigative endeavors underscore the versatile pharmacodynamic canvas upon which Loteprednol operates, inviting a broader discourse on its role in multidisciplinary ocular care. Patient adherence, a critical determinant of therapeutic success, appears to be enhanced by the drug’s relatively low dosing frequency and favorable tolerability profile, factors that collectively reduce the attrition observed with more aggressive regimens. In summation, while Loteprednol is not a panacea for all ocular inflammatory disorders, its introduction represents a judicious balance between efficacy and safety, embodying the progressive refinement of steroidal therapy in contemporary ophthalmology. Future longitudinal studies are warranted to delineate the long‑term outcomes associated with chronic Loteprednol usage, particularly in elderly cohorts susceptible to ocular comorbidities. Additionally, comparative cost‑effectiveness analyses will clarify its position relative to both generic steroids and newer biologic agents. Ultimately, the ongoing accumulation of real‑world evidence will shape clinical guidelines and inform patient‑centered decision making.
Jay Ram August 12, 2023
Wow, that was quite the essay on Loteprednol – I’m impressed by the dedication, even if I just needed a quick rundown.