When you hear "generic drug," you probably think of a cheaper version of your brand-name pill-same active ingredient, same effect, same price cut. But what about biosimilars? They’re also cheaper alternatives to expensive medications, but they’re not the same thing. Confusion between biosimilars and generics is common-even among doctors-and it can lead to misunderstandings about safety, substitution, and cost. Let’s cut through the noise and explain exactly how they differ, why it matters, and what it means for your treatment.
What Are Generics?
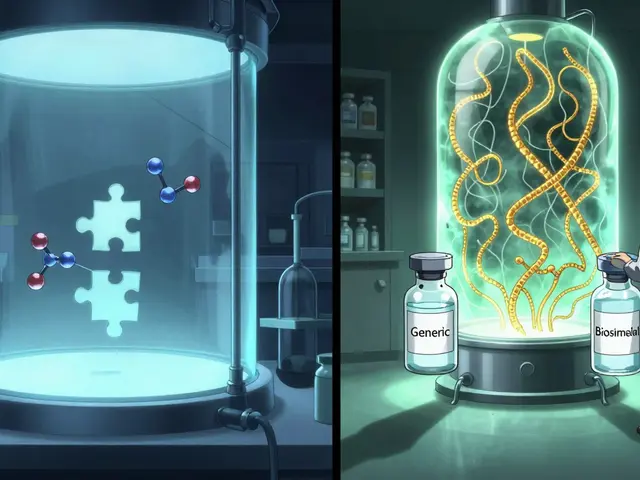
Generics are exact chemical copies of brand-name drugs. Think of aspirin, ibuprofen, or metformin. These are small molecules made in a lab using precise chemical reactions. Once the original patent expires, any manufacturer can produce the same formula, as long as it matches the brand-name drug in strength, dosage, safety, and how fast it gets into your bloodstream. The FDA requires generics to be bioequivalent-meaning they perform the same way in your body as the original. No guesswork. No variations. If you take a generic version of Lipitor, you’re getting the same molecule as the branded version, just without the marketing.Because the chemistry is simple and repeatable, making generics is cheap. Development costs are usually between $2 million and $5 million. That’s why generics cost 40% to 50% less than brand-name drugs. In the U.S., about 90% of all prescriptions are filled with generics. They’re everywhere-your local pharmacy, your insurance formulary, your Medicare Part D plan. And because they’re chemically identical, pharmacists can swap them in automatically without asking your doctor.
What Are Biosimilars?
Biosimilars are different. They’re not copies-they’re highly similar versions of complex biologic drugs. Biologics aren’t made in a beaker; they’re grown in living cells-like yeast, bacteria, or mammalian cells. These drugs are huge proteins, often thousands of times larger than small-molecule drugs. For example, Humira (adalimumab), used for rheumatoid arthritis, is a monoclonal antibody weighing about 148,000 daltons. Compare that to ibuprofen, which weighs just 206 daltons. The size alone makes replication impossible.Biosimilars are made using the manufacturer’s own cell line and process. Even if two companies try to copy the same biologic, their versions will have tiny differences-like natural variations in sugar chains or protein folding. These aren’t mistakes; they’re expected. The FDA says a biosimilar must show no clinically meaningful differences in safety, purity, or potency compared to the original. That means it works the same way in your body, but it’s not chemically identical.
Because of this complexity, developing a biosimilar costs $100 million to $200 million. That’s 50 times more than making a generic. And even then, you can’t just copy the original manufacturer’s process-you have to reverse-engineer it. That’s why biosimilars only save 15% to 33% off the brand-name price, not 50%.
Regulatory Pathways: Why One Is Simpler Than the Other
Generics follow the Hatch-Waxman Act of 1984. To get approval, a company must prove bioequivalence-usually through blood tests showing the drug is absorbed at the same rate and to the same level as the brand. No large-scale clinical trials needed. Just a few hundred patients, sometimes just healthy volunteers.Biosimilars follow the BPCIA of 2009. The process is step-by-step and intense. First, you analyze the structure of the original drug using dozens-if not hundreds-of lab tests. Then you test how it behaves in animals. Then you run clinical trials to prove it works the same in humans. You must show it doesn’t cause more immune reactions. You must prove it’s as safe over time. It’s not enough to say it’s "similar." You have to prove it’s not meaningfully different in any way that affects safety or effectiveness.
As of November 2023, the FDA has approved 42 biosimilars. Over 10,000 generics are on the market. The difference in volume isn’t just about cost-it’s about complexity.
Can Pharmacists Switch You Automatically?
This is where it gets personal. If your doctor prescribes a generic, your pharmacist can swap it in without telling you-or your doctor. That’s automatic substitution, allowed in all 50 states.With biosimilars? Not so fast. Only biosimilars that are designated as interchangeable can be swapped automatically. And as of early 2026, only 7 out of 42 approved biosimilars have that status. The rest require your doctor to specifically prescribe them by name.
Why the restriction? Because biologics can trigger immune responses. Even tiny changes in the protein structure might cause your body to react-especially if you’ve been on the original drug for years. Switching from Humira to a biosimilar might be fine for someone just starting treatment. But for someone stabilized on the brand, a switch could mean flare-ups, rashes, or worse. That’s why doctors are cautious.
Market Adoption: Why Generics Are Everywhere and Biosimilars Are Still Catching Up
Generics dominate because they’re simple, cheap, and trusted. They’ve been around for decades. Pharmacists know them. Patients know them. Insurance plans push them.Biosimilars are newer. They’re used mostly in specialty areas: rheumatology, oncology, diabetes. The big ones-Humira, Enbrel, Herceptin-cost tens of thousands of dollars a year. That’s where the savings matter most. Hospitals and specialty pharmacies are leading adoption. About 45% of U.S. hospitals now use at least one biosimilar.
But there’s resistance. Some doctors are still learning. Some patients are nervous. Some insurers don’t make it easy. In Europe, biosimilars make up 35% of the biologics market. In the U.S., it’s under 3%. Why? Patent thickets. Big pharma companies like AbbVie have filed hundreds of patents around Humira to delay competition. It took until 2023 for the first biosimilars to hit the market, even though the first one was approved in 2016.
That’s changing. The Inflation Reduction Act of 2022 is helping. It’s closing the Medicare Part D donut hole and lowering out-of-pocket costs for biologics. More biosimilars are coming. Amjevita, the first interchangeable Humira biosimilar, launched in January 2024 with a 35% discount. More are on the way for Stelara, Eylea, and others.
What This Means for You
If you’re taking a small-molecule drug-like a blood pressure pill, an antibiotic, or a diabetes tablet-you’re likely already on a generic. No worries. It’s safe, effective, and saves you money.If you’re on a biologic-like a shot for psoriasis, Crohn’s, or cancer-you might be offered a biosimilar. Ask your doctor: Is it interchangeable? Has it been studied in patients like me? What happens if I switch? You don’t have to switch. But if your doctor says it’s safe and your insurance saves you hundreds a month, it’s worth considering.
Don’t assume biosimilars are "generic biologics." They’re not. They’re a different category. They’re not cheaper because they’re easier to make-they’re cheaper because they’re competing in a market that used to be monopolized by one company. That competition is good. It’s just more complicated.
What’s Next?
The FDA is working on clearer guidelines for interchangeability, especially for complex biologics like antibody-drug conjugates. More biosimilars will come. More will get the interchangeable label. More patients will benefit from lower costs.But for now, remember this: Generics = exact copies. Biosimilars = highly similar, carefully studied, not identical. Both are safe. Both save money. But they’re not the same thing. And knowing the difference helps you make smarter choices-with your doctor, your pharmacist, and your wallet.





Ashley Porter January 24, 2026
Biosimilars are wild when you think about it-like trying to clone a snowflake and calling it identical. The FDA’s ‘no clinically meaningful differences’ standard is genius, honestly. It’s not about perfection, it’s about function. If your immune system doesn’t throw a fit, it’s good to go.
Uche Okoro January 25, 2026
Let’s not sugarcoat this: the regulatory asymmetry between generics and biosimilars is a structural artifact of 20th-century pharmacology meeting 21st-century biotech. Hatch-Waxman was designed for small molecules-molecular Lego blocks. Biosimilars require a systems biology approach: glycosylation profiles, Fc receptor binding kinetics, aggregation propensity. The $150M development cost? That’s the price of precision.
And don’t get me started on interchangeability. The FDA’s ‘extrapolation’ model is mathematically sound, but clinicians are terrified of off-label switching in chronic autoimmune patients. One misfolded glycan chain in a TNF-alpha inhibitor and you’ve got neutralizing antibodies. Welcome to the land of immunogenicity.
Meanwhile, generics? You can synthesize them in a garage lab with a fume hood and a budget. Biosimilars need clean rooms, bioreactors, and a PhD in protein chromatography. The 15-33% savings? That’s not a failure-it’s the cost of biological fidelity.
And yes, the patent thickets are criminal. AbbVie’s Humira patent portfolio reads like a legal thriller. But the market is finally cracking. Amjevita’s 35% discount? That’s the first domino. Next up: Stelara biosimilars in 2025. Expect formulary shifts in Medicare Advantage plans to accelerate adoption.
Bottom line: generics are chemistry. Biosimilars are biology. And biology, as we all know, is messy.
shivam utkresth January 26, 2026
Man, I remember when my cousin in Delhi got her rheumatoid arthritis meds switched to a biosimilar-saved her like $2k a year. But she was terrified at first, like, ‘Is this like fake medicine?’ You know what helped? Her doctor showing her the FDA’s comparison charts-side-by-side protein folding diagrams, the whole nine yards. It’s not magic, it’s science. And yeah, it’s expensive to make, but damn if it ain’t worth it.
Here’s the thing: in India, we’ve got generics for everything. But biosimilars? We’re catching up fast. Biocon’s biosimilars are in 80+ countries now. It’s not just about cost-it’s about access. A kid in rural Nigeria shouldn’t have to choose between insulin and rent.
So yeah, they ain’t generics. But they’re the closest thing we got to justice in pharma right now.
Kipper Pickens January 26, 2026
Let’s be real-the reason biosimilars aren’t everywhere isn’t because they’re unsafe. It’s because the pharmaceutical industry built an entire business model around monopolizing biologics. The ‘complexity’ argument? That’s just a smokescreen for profit retention. The same companies that spent $200M to reverse-engineer Humira also spent $500M on lobbying to delay competition.
And yet, we’re still acting like biosimilars are risky. Meanwhile, a patient on a brand-name biologic gets a $10,000 bill every month. The real danger isn’t the biosimilar-it’s the system that lets one company charge that much for a protein.
Also, ‘interchangeable’ status isn’t a safety rating. It’s a legal checkbox. The FDA doesn’t say ‘this is safer’-they say ‘this can be swapped without the prescriber’s consent.’ Big difference.
Stop treating biosimilars like second-class drugs. They’re just the next evolution of competition. And competition? That’s the only thing that lowers prices in healthcare.
Aurelie L. January 26, 2026
So… biosimilars are like… kinda similar? But not really? And you can’t just swap them? And it costs a fortune? And we’ve had them since 2016 but only 7 are interchangeable? And the drug companies are still rich? Cool. Cool cool cool.
Joanna Domżalska January 27, 2026
Everyone’s acting like biosimilars are some breakthrough. Newsflash: they’re just cheaper versions of drugs that cost $200k a year because the FDA let one company patent a protein sequence. The real problem isn’t biosimilars-it’s that we let corporations patent biology. You can’t patent a gene. But you can patent a protein made from it? That’s not science. That’s capitalism.
Also, why are we still using monoclonal antibodies for everything? Maybe we should be funding gene therapies instead of playing copycat with proteins. Biosimilars are just delaying the real innovation.
Sally Dalton January 28, 2026
OMG I JUST LEARNED SO MUCH 😭 Like I thought biosimilars were just generics but for fancy shots?? But nooo they’re like… the cousin who looks super similar but has a totally different personality?? And the fact that you can’t just swap them like a generic?? That’s wild. I’m on a biologic for my psoriasis and my doc just said ‘we’ll talk about biosimilars next visit’ and I was like ‘is it safe??’ and now I feel way better about it. Also I just looked up Amjevita and it’s like 35% off?? That’s like a free vacation!! Thank you for explaining this so clearly!! 🥹💖
Shawn Raja January 29, 2026
So let me get this straight: generics are like buying a knockoff iPhone case that fits perfectly. Biosimilars are like hiring a team of scientists to 3D-print the original iPhone… using different plastic, different glue, and a slightly different color. Then the FDA says, ‘It works the same, go ahead.’
Meanwhile, the original iPhone maker spent $10 billion on R&D, then sued everyone who even looked at the blueprint. And now we’re supposed to be grateful for a 35% discount on a $100k drug? Welcome to American healthcare, folks. Where innovation is monetized, and savings are treated like a gift.
Also, why are we still using biologics for everything? Maybe we should’ve invested in CRISPR instead of copying proteins. But hey, at least we’ve got a biosimilar for that.
Ryan W January 29, 2026
Foreign-made biosimilars are a national security risk. The U.S. spends billions on biologics while China and India churn out knockoffs under the guise of ‘affordable medicine.’ We need to protect our domestic biotech industry-not subsidize foreign competition under the banner of ‘access.’
And why are we letting pharmacists swap drugs without a doctor’s approval? That’s how people die. The FDA’s ‘interchangeable’ label is a joke. If it’s so similar, why does it take 10 years and $200M to prove it?
America doesn’t need cheaper drugs. We need better drugs. Made here. By Americans.
TONY ADAMS January 29, 2026
Bro I just got my Humira biosimilar and my insurance paid $10. I’m not even mad anymore. Just glad I don’t have to sell a kidney.
Ashley Karanja January 31, 2026
Okay so I just spent 45 minutes reading this and I’m crying? Not because it’s sad-because it’s so beautifully explained. Like… I’ve been on a biologic for 7 years and I thought biosimilars were just ‘generic biologics’ and I was terrified to switch. But now I get it: it’s not about copying-it’s about matching function. The glycosylation, the folding, the immunogenicity risk… it’s like your DNA isn’t exactly like your twin’s, but you still both breathe the same air.
And the fact that only 7 are interchangeable?? That’s not a flaw-that’s caution. We’re talking about people’s immune systems here. Not pills. Not chemicals. Living systems. I’m so glad someone finally broke this down without jargon overload. Also-Amjevita? I’m asking my doc next week. And yes, I’m sharing this with my support group. This is the kind of info that saves lives. Thank you. 🤍
Ashley Porter February 1, 2026
Actually, I just checked-the first interchangeable biosimilar for Stelara just got approved last week. So that number’s going up fast. The tide’s turning.