The Purple Book isn’t a novel you read for fun-it’s the U.S. Food and Drug Administration’s official, searchable database that tells you which biological medicines are approved, which ones are biosimilars, and which ones can be swapped out like generics. If you’re a pharmacist, doctor, or even a patient trying to understand why your insulin prescription changed, the Purple Book is the place to look. It’s not just a list. It’s a decision-making tool that affects how medicines are dispensed, prescribed, and paid for across the country.
What Exactly Is the Purple Book?
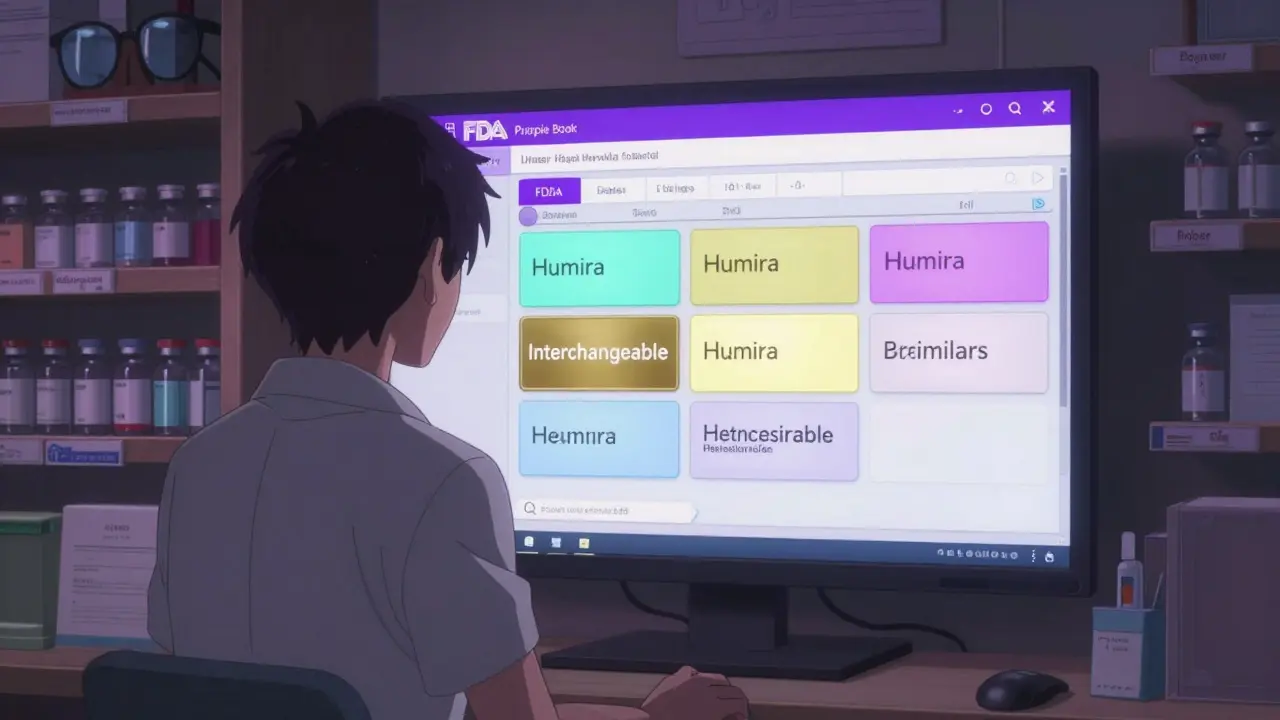
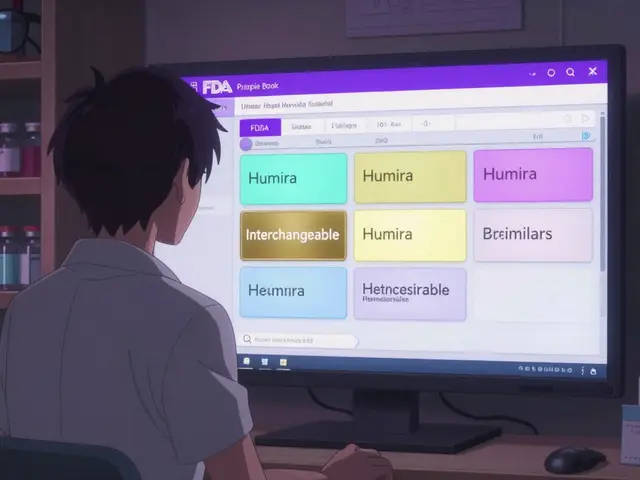
The Purple Book is the FDA’s public database for all licensed biological products in the United States. That includes everything from insulin and rheumatoid arthritis drugs to cancer treatments and vaccines. It’s not just about the original brand-name biologics-it’s also about their copies. These copies aren’t called generics like with pills. They’re called biosimilars. And some of them? They’re interchangeable. Before 2020, the FDA kept two separate lists-one for drugs handled by its drug center and another for biologics handled by its biologics center. That made it confusing. Now, it’s one clean, searchable database. You can type in a drug name like Humira or Lantus, and the Purple Book will show you the original product, any biosimilars that match it, and which ones are approved as interchangeable. The system uses color-coded cards: if two products have the same color, they’re linked. One card might show the original, and others show the biosimilars that copy it.Biosimilars vs. Interchangeable Products: The Key Difference
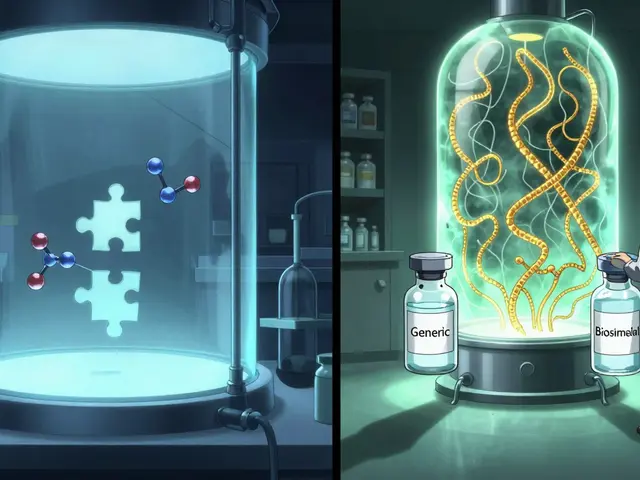
Here’s where things get tricky. All interchangeable products are biosimilars. But not all biosimilars are interchangeable. Think of it like this: a biosimilar is a close copy. It has to match the original in safety, purity, and how well it works. The FDA requires extensive testing to prove there are no clinically meaningful differences. An interchangeable product goes further. It has to prove that switching back and forth between the original and the copy won’t cause extra risk or reduce effectiveness. That means if a patient takes the brand-name drug for three months, then switches to the biosimilar for three months, then switches back-their condition stays stable. No flare-ups. No unexpected side effects. That’s the bar for interchangeability. The FDA doesn’t say interchangeable biosimilars are better. They’re just proven to be switchable without harm. This distinction matters because only interchangeable products can be automatically substituted by a pharmacist without the doctor’s permission-assuming state laws allow it.How the FDA Determines Interchangeability
Getting an interchangeable designation isn’t just about showing similarity. It requires additional clinical studies. These are called switching studies. Companies have to prove that multiple switches between the reference product and the biosimilar don’t change the patient’s response. For example, if someone with Crohn’s disease is on a reference biologic and then switches to a biosimilar, then switches back, then switches again-their disease activity must remain controlled. No drop in effectiveness. No spike in antibodies. No new side effects. The FDA’s guidance says these studies must show the risk of switching is no greater than using the original drug alone. That’s a high standard. It’s why, as of late 2023, only seven biosimilars had earned the interchangeable label. Two are insulins. Three treat inflammatory conditions like rheumatoid arthritis. Two are for eye diseases. That’s it. More are coming, but the process is slow because the evidence has to be rock solid.
Why State Laws Matter More Than You Think
Even if the FDA says a biosimilar is interchangeable, that doesn’t mean a pharmacist can swap it automatically. Each state has its own rules. As of 2023, 47 states and Puerto Rico allow pharmacists to substitute an interchangeable biosimilar without contacting the doctor. But that’s not universal. Some states require the pharmacist to notify the prescriber. Others require the patient to be informed in writing. A few still require the doctor to specifically authorize substitution. This patchwork of laws creates real-world confusion. A patient in Texas might get a substituted biosimilar without knowing. A patient in New York might be given the brand-name drug even if the interchangeable version is available-because the pharmacist wasn’t allowed to switch. The Purple Book tells you what the FDA approved. But it doesn’t tell you what your local pharmacy can do. The FDA has made it clear: interchangeability is a federal designation. Substitution is a state-level decision. That’s why pharmacists rely on the Purple Book-not just to know what’s approved, but to understand what they’re legally allowed to do.What You’ll Find in a Purple Book Entry
Each product listing includes key details. First, the date it was licensed under section 351(a) of the Public Health Service Act-that’s the original brand. Then, if there’s a biosimilar, it’s marked as 351(k). If it’s interchangeable, it says so clearly. The entry also shows if the original product still has exclusivity-meaning no biosimilars can be approved yet. Some drugs have 12 years of market protection. Icons show how the drug is delivered: autoinjector, pre-filled syringe, vial. That helps prescribers and pharmacists know what’s available. You’ll also see the brand name and the generic name. The database doesn’t just list products-it connects them. Click on a reference product, and you’ll see all its biosimilar and interchangeable copies grouped underneath.What the Purple Book Doesn’t Tell You
The Purple Book doesn’t include pricing. It doesn’t say which product is cheaper. It doesn’t tell you what insurance covers. It also doesn’t list unbranded biologics-those are copies that the FDA considers equivalent but haven’t gone through the formal biosimilar approval process. They’re not interchangeable, and they’re not labeled as biosimilars. So if you see a drug with no brand name but the same active ingredient, check carefully. It might not be in the Purple Book at all. The database also doesn’t explain why one biosimilar was approved as interchangeable and another wasn’t. That’s buried in the FDA’s review documents, which aren’t publicly summarized. You need to dig into the official approval letters to understand the full reasoning.
Who Uses the Purple Book-and Why
Pharmacists use it daily to know what they can substitute. Prescribers use it to understand their options when a patient asks for a cheaper alternative. Payers-insurance companies and Medicare-use it to set formularies. Patients? They should use it too. If you’re on a biologic that’s expensive and your doctor mentions a biosimilar, you can look it up yourself. See if it’s interchangeable. See if your state allows substitution. That knowledge puts you in control. The Purple Book was created to bring transparency to a complex market. Before 2010, biologics had no path for copies. They were too complex to replicate. The BPCIA changed that. But the complexity didn’t disappear. The Purple Book is the FDA’s answer: clear, public, and updated regularly.What’s Next for the Purple Book?
More biosimilars are coming. More will seek interchangeability. The FDA is updating its labeling guidelines to make sure product names and information are clear-so patients and providers aren’t confused. Companies are investing in switching studies because they know interchangeability opens the door to wider use. But the biggest challenge isn’t science. It’s adoption. Even if a biosimilar is approved as interchangeable, doctors may still prescribe the brand name out of habit. Pharmacists may hesitate because of state rules. Patients may fear switching. The Purple Book gives the facts. The rest-trust, education, policy-depends on people.Is the Purple Book only for U.S. patients?
Yes. The Purple Book is maintained by the U.S. Food and Drug Administration and only includes biological products approved for sale in the United States. Other countries have their own systems-for example, the European Medicines Agency publishes a similar list for biosimilars approved in the EU, but it’s separate and uses different criteria.
Can I substitute a biosimilar without a doctor’s approval?
Only if the biosimilar has an FDA interchangeability designation and your state allows pharmacist substitution. Even then, some states require the pharmacist to notify the prescriber or document the swap. Always check your state’s pharmacy laws. You can’t assume substitution is automatic just because a product is labeled interchangeable.
Are interchangeable biosimilars safer than non-interchangeable ones?
No. The FDA states clearly that interchangeability doesn’t mean the product is safer or more effective. It only means that switching between it and the original product has been proven to carry no additional risk. Both types meet the same high bar for similarity. The only extra requirement for interchangeability is proof that multiple switches don’t harm the patient.
How do I find out if my medication is in the Purple Book?
Go to the FDA’s Purple Book website and search by brand name, generic name, or active ingredient. The database is free and searchable. If your drug is listed as a 351(a) reference product, a 351(k) biosimilar, or a 351(k) interchangeable product, it’s included. If it’s not there, it hasn’t been approved under the biosimilar pathway.
Why are so few biosimilars interchangeable?
Because proving interchangeability requires more clinical data than proving biosimilarity. Companies must run switching studies that show repeated changes between the reference and biosimilar don’t affect safety or effectiveness. These studies are expensive and time-consuming. Many manufacturers choose to seek only biosimilar approval first, which is easier and faster. Interchangeability is a strategic decision-not a scientific necessity.
Does the Purple Book include biosimilars from other countries?
No. The Purple Book only includes products approved by the U.S. FDA. A biosimilar approved in Canada, the EU, or Japan won’t appear unless it’s also submitted to and approved by the FDA for sale in the United States. Global approvals don’t transfer.
Can I use the Purple Book to compare prices?
No. The Purple Book lists approval status, product names, and designations-but not pricing, availability, or insurance coverage. You’ll need to check with your pharmacy, insurer, or a drug pricing tool like GoodRx for cost information.
Are biosimilars as effective as the original biologics?
Yes. Every biosimilar approved by the FDA must demonstrate there are no clinically meaningful differences in safety, purity, and potency compared to the original. This is based on extensive analytical, preclinical, and clinical data. Patients who switch from a brand-name biologic to an approved biosimilar should expect the same results.



Mark Alan January 28, 2026
This Purple Book is literally the only thing keeping my insulin from costing $1,200 a month 😭 THANK YOU FDA. If you're still paying full price for biologics, you're being robbed. 🇺🇸💪
Linda O'neil January 30, 2026
If you're a patient, don't just take your doctor's word for it-go check the Purple Book yourself. I showed my mom how to use it last week and she switched to an interchangeable biosimilar for her RA meds. Saved her $800/month. Knowledge is power, folks!
Chris Urdilas February 1, 2026
So let me get this straight-we spent 12 years making biologics untouchable by generics, then created this whole 'biosimilar' loophole just so Big Pharma can keep charging $20K a year... and now we're celebrating a database that doesn't even tell us the price? 🤡 The system is rigged, but at least we got emojis.
Howard Esakov February 1, 2026
The fact that you need a PhD in regulatory policy just to understand whether your pharmacy can swap your Humira is a national disgrace. The FDA’s Purple Book is a beautifully designed tombstone for American healthcare innovation. 🏛️
Meanwhile, in Europe, they’ve had biosimilar adoption rates over 80% for a decade. We’re still arguing about whether a pharmacist can *look* at the database.
Lance Long February 3, 2026
I know this sounds like a boring government doc, but honestly? This could save your life-or your wallet. I’ve seen patients cry because they couldn’t afford their biologic. Then they found an interchangeable biosimilar and started breathing again. Don’t sleep on this. Your body deserves better.
Timothy Davis February 5, 2026
The 7 interchangeable biosimilars? That’s not progress-that’s a failure of incentive structure. The switching studies cost $50M+ and take 5 years. Meanwhile, the originators extend exclusivity through patent thickets. This isn’t science-it’s corporate chess. And we’re the pawns.
John Rose February 5, 2026
I’ve been using the Purple Book for years as a pharmacy resident. It’s the single most underused tool in healthcare. If you’re a provider, teach your patients how to use it. If you’re a patient, demand your pharmacist show you the entry. Transparency isn’t optional-it’s a right.
Colin Pierce February 6, 2026
My cousin switched from Enbrel to an interchangeable biosimilar and hasn’t had a flare in 18 months. Same dose, same results, 70% cheaper. People think biosimilars are 'inferior'-they’re not. They’re just cheaper versions of something that was overpriced to begin with.
James Dwyer February 7, 2026
I didn’t know this existed until my doctor mentioned it. Now I check it every time my prescription renews. It’s like having a cheat code for healthcare. No fluff. No ads. Just facts. This is what public health should look like.
jonathan soba February 9, 2026
The fact that 47 states allow substitution but the FDA doesn’t mandate it is a regulatory nightmare. We have federal approval but state-level chaos. This isn’t healthcare-it’s a patchwork quilt of legal ambiguity. Someone needs to fix this.
matthew martin February 9, 2026
The Purple Book is the unsung hero of American biologics. It’s like Google Maps for medicine-except instead of traffic, it shows you which drugs can swap without blowing up your immune system. 🗺️💉
And yeah, it’s boring as hell to read… but if you’ve ever had to choose between rent and insulin? You’ll read it twice.
Jeffrey Carroll February 11, 2026
The regulatory framework surrounding biosimilars is one of the most sophisticated public health tools developed in the last decade. While the public discourse remains polarized, the scientific consensus on biosimilar equivalence is robust and well-documented. The Purple Book is a model of transparency.
doug b February 12, 2026
Look. You don’t need to be a doctor to get this. If your drug has a purple book entry with 'interchangeable' next to it? You can ask your pharmacist to swap it. No big deal. No paperwork. Just cheaper medicine. Stop overcomplicating it.
Katie Mccreary February 12, 2026
I bet the people who made this database never had to pay $15K for a shot. Meanwhile, I’m choosing between my meds and my kid’s school trip. So thanks, I guess.
Mark Alan February 13, 2026
^^^ you’re not alone. I’ve been there. That’s why I fight for this stuff. The Purple Book isn’t perfect-but it’s the only thing standing between you and bankruptcy. Use it. Share it. Demand better.