Biosimilars: What They Are, How They Work, and Why They Matter
When you hear biosimilars, highly similar versions of complex biologic drugs made after the original patent expires. Also known as biologic generics, they offer the same clinical benefits as the original but at a fraction of the cost. Unlike regular generic pills, biosimilars aren’t exact copies—they’re made from living cells, not chemicals, so they’re more like identical twins than clones. The FDA requires them to match the original in safety, purity, and potency, but they’re not interchangeable unless specifically approved as such.
Biosimilars exist because biologics, complex drugs made from living organisms like proteins or antibodies—used for cancer, rheumatoid arthritis, diabetes, and more—can cost over $100,000 a year. Companies like Amgen and Pfizer now make biosimilars for drugs like Humira and Enbrel, cutting prices by 15% to 40%. This isn’t just about savings—it’s about access. Many patients couldn’t afford these drugs before biosimilars arrived. But not all biosimilars are created equal. Some are approved for the exact same uses as the original; others only for a few. Always check the label.
Switching from a brand-name biologic to a biosimilar isn’t always simple. Some doctors worry about immune reactions or subtle differences in how the body responds, even though studies show no meaningful difference in outcomes. If you’re on a biologic and your pharmacy switches you to a biosimilar, track how you feel. Watch for new side effects, flare-ups, or changes in energy. Your doctor may need to run blood tests or adjust your dose. And don’t assume all biosimilars are cheaper—some are priced almost the same as the original, especially early on. Look for therapeutic equivalence, when a biosimilar performs the same way as the original in real-world use and ask your pharmacist if it’s approved for substitution.
Behind the scenes, patent battles and legal loopholes delay biosimilar entry. The Hatch-Waxman Act, a U.S. law that balances innovation and competition in drug markets was designed for small-molecule drugs, not biologics. So biosimilars face longer exclusivity periods and more legal hurdles. That’s why you’ll see more biosimilars hitting the market now—patents are finally expiring on big sellers like Humira. But until then, manufacturers use tricks like tweaking dosing schedules or adding new indications to extend their monopoly.
What you’ll find in the posts below isn’t just theory—it’s real-world guidance. You’ll learn how to track if your generic medication is working after a switch, how manufacturer savings programs can cut brand drug costs, and why authorized generics sometimes drop prices faster than first-to-file versions. There’s also deep dives into how AI is now helping pharmacies recommend personalized generics based on your genes, and how digital tools help patients stick to their meds. None of this is guesswork. These are the tools, tricks, and traps you’ll face when navigating today’s complex drug system—and how to come out ahead.
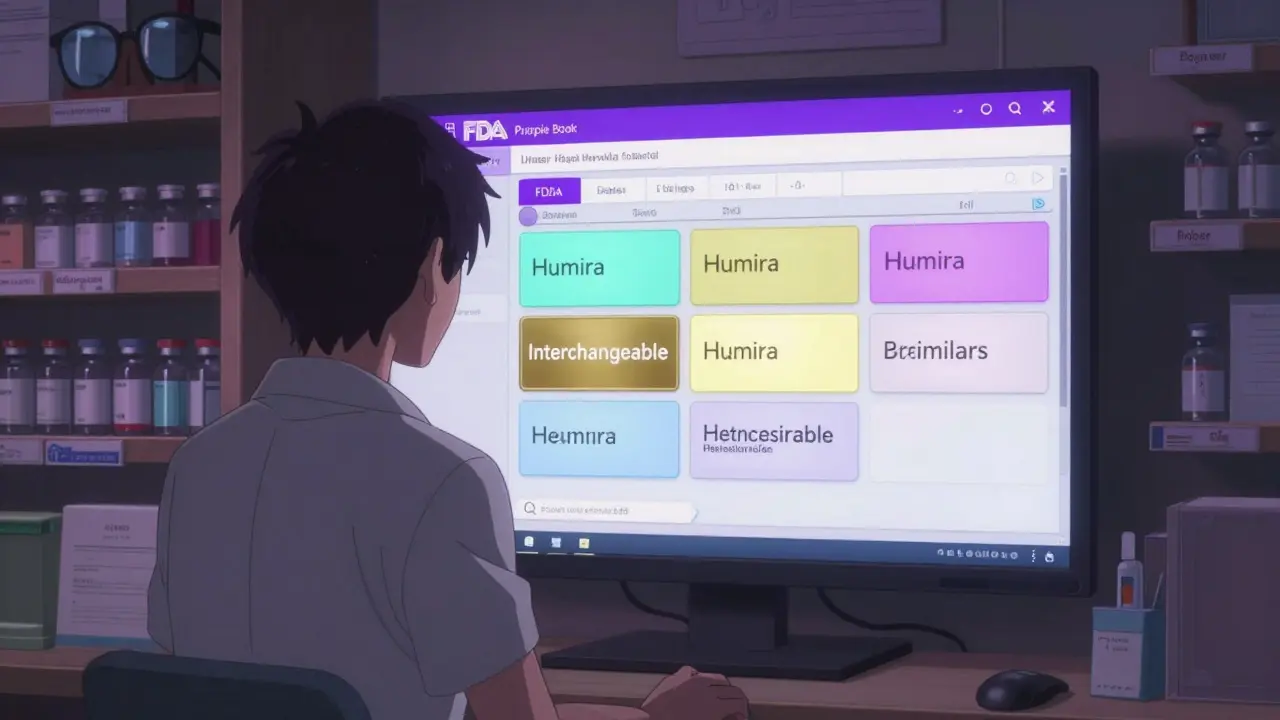
The Purple Book: Understanding Biosimilars and Interchangeability from the FDA
The FDA's Purple Book is the official guide to biosimilars and interchangeable biological products in the U.S. Learn how it works, what interchangeability really means, and why state laws affect whether you can switch medications.
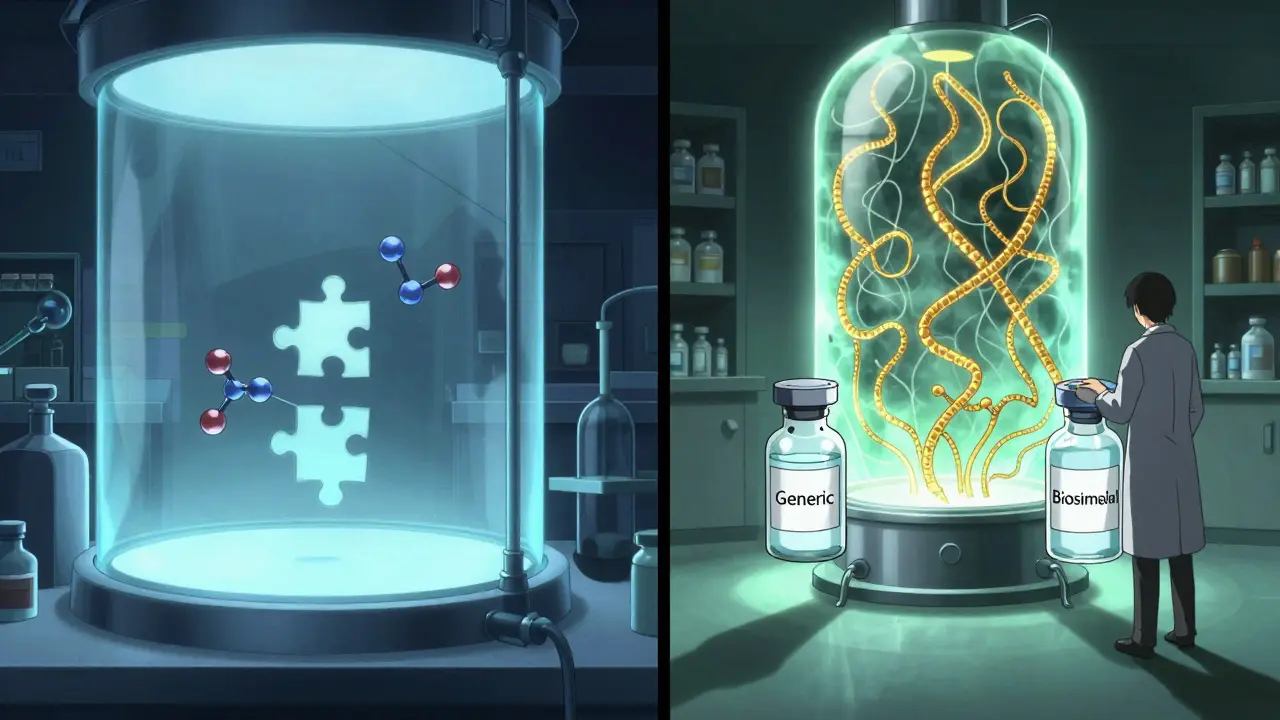
Biosimilars vs Generics: Key Differences Explained Simply
Biosimilars and generics both lower drug costs, but they’re not the same. Generics are exact chemical copies; biosimilars are complex, highly similar versions of biologic drugs. Learn how they differ in manufacturing, regulation, cost, and substitution rules.
Future of Global Generic Markets: Key Trends and Predictions for 2025-2030
The global generic drugs market is evolving fast. With biosimilars rising, supply chains under pressure, and emerging markets driving growth, generics remain essential for affordable healthcare-but only for those who adapt.